The federal government through the FDA sets forth the rules and regulations (called MQSA) that control the quality and standards for the performance of mammograms. This screening test is USELESS unless the test results are properly reported so that any necessary action or follow up can be taken. A letter is to be sent to patients and the full report to physicians when referring or other physicians are provided to the mammogram facility. The patient’s test results should be received and REVIEWED by their physician. The facility, the radiologist and patient’s physician have the non-delegable duty to follow MQSA rules and physician standards to review, review and report, it is simply smart to take an active role in your medical treatment and follow up on your tests.
In 2009, we settled a suit against a hospital and physician’s office relating to the mishandling of an abnormal mammogram report that resulted in a two year delay in treatment of a Stage I breast cancer. The cancer developed to a Stage III(b) tumor with metastatic by the time it was discovered. The case was hotly contested on the issues of negligence, comparative fault, causation, damages and injury and prognosis from the delay. There was finger pointing between the physician’s office and hospital mammogram center. FDA rules and radiological standards were critical to the outcome of the case. The case settled for over $650,000.00. Happily our client is still cancer free over eight years from diagnosis.
According to the CDC, CDC Link
In 2009 (the most recent year numbers are available)—
- 211,731 women in the United States were diagnosed with breast cancer.
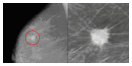
Suspect Mammogram Image
- 40,676 women in the United States died from breast cancer.
Over 15% of all mammography centers had violations when inspected by the FDA according to 2013 FDA figures. FDA inspection results
Mammography results reporting requirements are set forth by law. See Section 900.12 (c) of the MQSA (Mammography Quality Standards Act-Regulations). MQSA REGULATIONS If the standards are not followed for reporting to patient’s physician and to the patient, a dangerous delay in treatment and decision making can occur.
In our case the defendant raised the 2 year statute of limitations. The patient wasn’t even aware of the suspect test within two years. We were able to prove that the facility had glitches in their patient reporting computer system, but the facility was able to produce some evidence that the facility did send the physician a report. The physician denied this, but we were able to demonstrate what we deemed problems in their receiving and reviewing of reports, including negligent officer staff practices. We were able to avoid the statute of limitations by using a statute that expands the short statute for the failure to report test results. The case settled shortly prior to trial after all expert depositions were taken.
Leave a Reply